
本课题组致力于有机反应方法学的开发,并努力推动新兴的有机反应在药物中间体以及功能材料中的应用。目前,课题组主要研究重排反应,特别是借助高价硫/碘化合物构建不稳定的重排前体,发展传统重排难以实现的重排过程。
我们的研究理念是融汇物理或者经济学中归纳和演绎的模型化思维,运用于同样依赖模型思维的有机化学,反之亦然。我们的目标是让每一位成员都能在科研的创新中找到自己的思维特质并把她发挥出来,享受激动人心的探索、理解、创造的过程。热烈欢迎喜爱有机化学的同学们加入我们的团队,我们将努力为大家营造良好的学习环境和宽松自由的科研氛围。欢迎优秀的研究生来课题组攻读博士学位。
地址:浙江师范大学11幢415室
邮箱:pengbo@zjnu.cn
团队成员:
彭勃(课题组负责人 教育部“青年长江学者”)
2000-2004 南京理工大学本科
2004-2010 大连理工大学(精细化工国家重点实验室)博士(导师:包明 教授)
2011-2013 德国马普煤炭研究所博士后(导师:NunoMaulide 教授)
2013-2014 美国伊利诺伊大学香槟分校研究助理(导师:Scott E. Denmark 教授)
2015年至今 浙江师范大学 教授
人才计划及获奖情况:
教育部青年长江学者
浙江省钱江学者特聘教授
浙江省新世纪151人才工程第一层次
院士结对培养青年英才计划
浙江省杰出青年基金获得者
浙江省万人计划青年拔尖人才
Thieme Chemistry Journals Award
张磊(副教授, 博士毕业于中国科学院生态环境研究中心, 导师:杜宇国研究员)
黄鑫(副教授, 博士毕业于美国麻省大学波士顿分校, 导师: Prof. Wei Zhang)
在读硕士研究生:
2020级:陈书升、李瑞琦、张宗卫、周丽江
2021级:樊锁江、黄子懿、茹莉莹、王欢欢、王梦瑶
2022级:刘冰杰、刘开瑞、万坤、叶晟、张理明、朱梦娇
已毕业硕士研究生:
2014级:尚利(优秀毕业生)、李晓锦(优秀毕业生)
2015级:罗凡、孙艳(优秀毕业生)
2016级:田俊菘(优秀毕业生)、何佳妮、赵伟钊
2017级:包王镇、陈孟源、胡梦杰、严超、张亚鸽(优秀毕业生)
2018级:李东阳、詹娅玲、张其峰、王明慧
2019级:董涛涛、刘艳萍(优秀毕业生)、潘文静、梁微健
独立以来研究论文: “+”为共同一作 “*”为通讯作者
(24) Revisiting Aromatic Claisen Rearrangement Using Unstable Aryl Sulfonium/Iodonium Species: The Strategy of Breaking Up the Whole into Parts. Yuchen Liang, Bo Peng* Acc. Chem. Res. 2022, 55, 2103. (Invited Review Article)
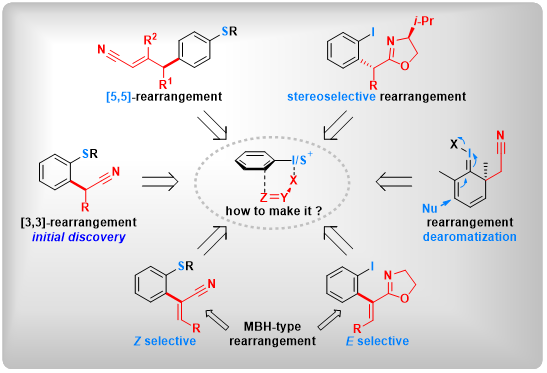

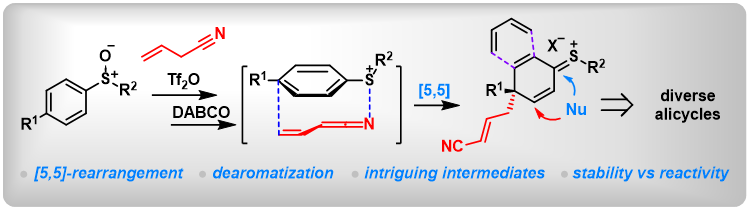
(23) Dearomative di- and trifunctionalization of aryl sulfoxides via [5,5]-rearrangement. Mengjie Hu,+ Yanping Liu,+ Yuchen Liang,+ Taotao Dong, Lichun Kong, Ming Bao, Zhi-Xiang Wang,* Bo Peng* Nat Commun 2022, 13, 4719.
(22) Sulfur(IV)-mediated umpolung α-heterofunctionalization of 2-oxazolines. Qifeng Zhang,+ Yuchen Liang,+ Ruiqi Li, Ziyi Huang, Lichun Kong, Peng Du, Bo Peng*Chem. Sci. 2022,13, 5164.
(21) Morita-Baylis-Hillman Type [3,3]-Rearrangement: Switching from Z- to E-selective α-Arylation by New Rearrangement Partners. Lei Zhang,+ Wangzhen Bao,+ Yuchen Liang,+ Wenjing Pan, Dongyang Li, Lichun Kong, Zhi-Xiang Wang,* Bo Peng* Angew. Chem. Int. Ed.2021, 60, 11414.
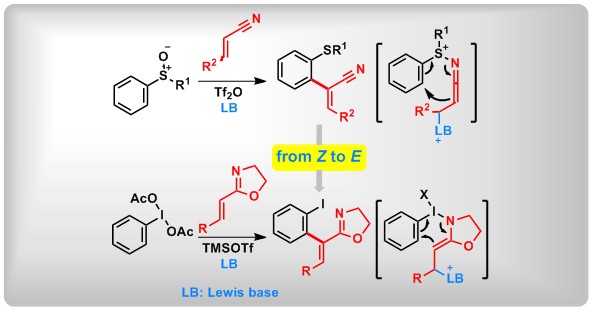
(20) Z-Selective α-Arylation of α,β-Unsaturated Nitriles via [3,3]-Sigmatropic Rearrangement. Mengyuan Chen,+ Yuchen Liang,+ Taotao Dong, Weijian Liang, Yanping Liu, Yage Zhang, Xin Huang, Lichun Kong, Zhi-Xiang Wang,* Bo Peng*Angew. Chem. Int. Ed.2021, 60, 2339.
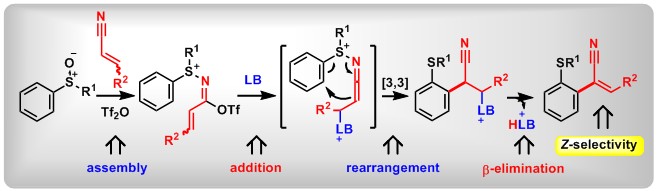
(19) Asymmetric Iodonio-[3,3]-Sigmatropic Rearrangement to Access Chiral α-Aryl Carbonyl Compounds. Junsong Tian,+ Fan Luo,+ Qifeng Zhang, Yuchen Liang, Dongyang Li, Yaling Zhan, Lichun Kong, Zhi-Xiang Wang,* Bo Peng* J. Am. Chem. Soc., 2020, 142, 6684.(Inside Cover)
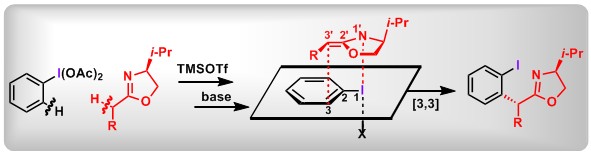

(18) Dearomatization of Aryl Sulfoxides: A Switch between Mono- and Dual-Difluoroalkylation. Xin Huang,+ Yage Zhang,+ Weijian Liang, Qifeng Zhang, Yaling Zhan, Lichun Kong, Bo Peng* Chem. Sci., 2020, 11, 3048.
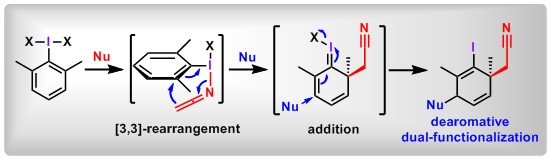
(17) Dearomative Dual-Functionalization of Aryl Iodanes. Weizhao Zhao,+ Xin Huang,+ Yaling Zhan, Qifeng Zhang, Dongyang Li, Yage Zhang, Lichun Kong, Bo Peng*Angew. Chem. Int. Ed.2019, 25, 17210.(Highlighted by X-mol).
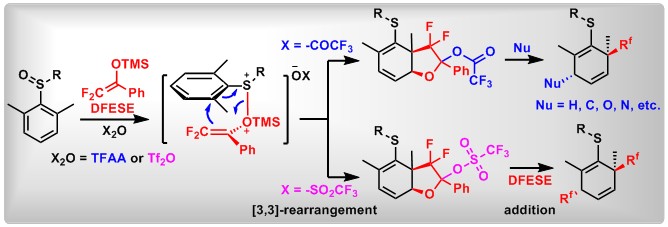
(16) The ortho-Difluoroalkylation of Aryliodanes with Enol Silyl Ethers through a Rearrangement Enabled by a Fluorine Effect. Xin Huang,+ Yage Zhang,+ Chaoshen Zhang,+ Lei Zhang, Ying Xu, Lichun Kong, Zhi-Xiang Wang,* Bo Peng*. Angew. Chem. Int. Ed. 2019, 58, 5956.
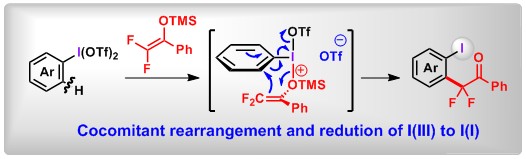
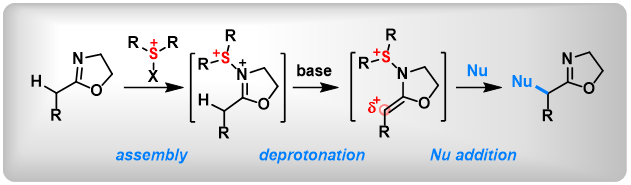
(15) [3,3] and [5,5]-Sigmatropic Rearrangement of Aryl Sulfoxides Using An “Assembly/Deprotonation” Technology. Lei Zhang, Mengjie Hu, Bo Peng* Synlett., 2019, 30, 2203. (Invited Synpact Article)
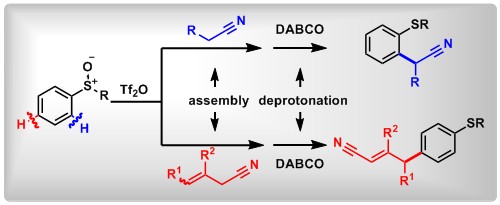
(14) Selective [5,5]-Sigmatropic Rearrangement by Assembly of Aryl Sulfoxides with Allyl Nitriles. Lei Zhang,+ Jia-Ni He,+ Yuchen Liang,+ Mengjie Hu, Li Shang, Xin Huang, Lichun Kong, Zhi-Xiang Wang,* Bo Peng*. Angew. Chem. Int. Ed. 2019, 58, 5316. (Highlighted by Synpacts and X-mol).
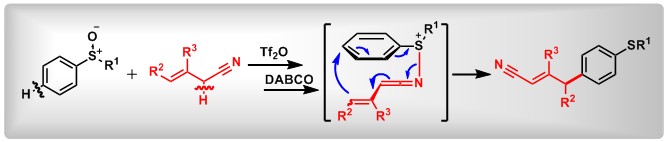
(13) Selective ortho C-H Cyanoalkylation of (Diacetoxyiodo)arenes via [3,3]-Sigmatropic Rearrangement. Junsong Tian, Fan Luo,+ Chaoshen Zhang,+ Xin Huang,* Yage Zhang, Lei Zhang, Lichun Kong, Xiaochun Hu, Zhi-Xiang Wang*, Bo Peng* Angew. Chem. Int. Ed.2018, 57, 9078.
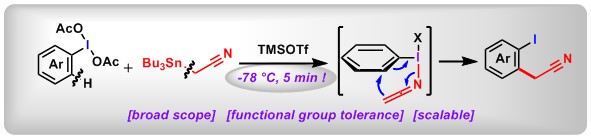
(12) Redox-Neutral α-Arylation of Alkyl Nitriles with Aryl Sulfoxides: A Rapid Electrophilic Rearrangement. Li Shang,+ Yonghui Chang,+ Fan Luo, Jia-Ni He, Xin Huang, Lei Zhang, Lichun Kong, Kaixiao Li, Bo Peng* J. Am. Chem. Soc., 2017, 139, 4211. (Highlighted by Synform 2017/07, A112–A114 and X-mol).
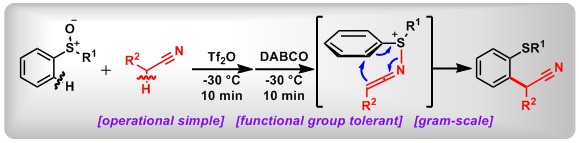
(11) α-C–H difluoroalkylation of alkyl sulfoxides via intermolecular Pummerer reaction. Xin Huang,+ Weizhao Zhao,+ Yuchen Liang,+ Minghui Wang, Yalin Zhan, Yage Zhang, Lichun Kong, Zhi-Xiang Wang,* Bo Peng*Org. Chem. Front.2021, DOI: 10.1039/d0qo01513j.
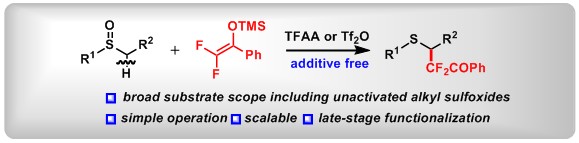
(10) [3,3]-Sigmatropic Rearrangement of Aryl Fluoroalkyl Sulfoxides with Alkyl Nitriles. Mengjie Hu, Jia-Ni He, Yanping Liu, Taotao Dong, Mengyuan Chen, Chao Yan, Yulu Ye, Bo Peng *Eur. J. Org. Chem.2020, 193. (YourJOC Talents)
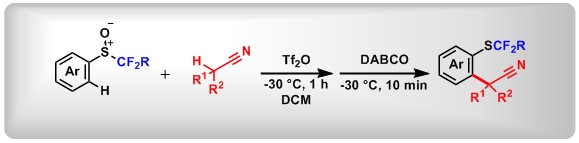
(9) Desulfurization of Diaryl(heteroaryl) Sulfoxides with Benzyne. De-Li Chen,+ Yan Sun,+ Mengyuan Chen, Xiaojin Li, Lei Zhang, Xin Huang, Yihui Bai, Fang Luo, Bo Peng*Org. Lett. 2019, 21, 3986.
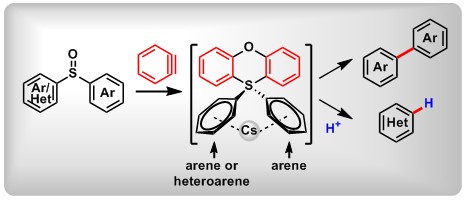
(8) Benzyne-mediated trichloromethylation of chiral oxazolines. Xin Huang,+Weizhao Zhao,+ De-Li Chen, Yaling Zhan, TingtingZeng, Huiquan Jin, Bo Peng*Chem. Commun., 2019, 55, 2070.
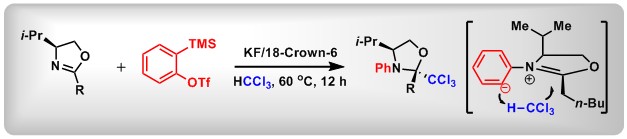
(7) Reductive ortho C-H Cyanoalkylation of Aryl(Heteroaryl) Sulfoxides: A General Approach to α-Aryl(Heteroaryl) Nitriles. Luo, F.; Lu, Y.; Hu, M.; Tian, J.; Zhang, L.;* Bao, W.; Yan, C.; Huang, X.; Wang, Z.-X.;* Peng, B.* Org. Chem. Front., 2018, 5,1756. (inside cover)
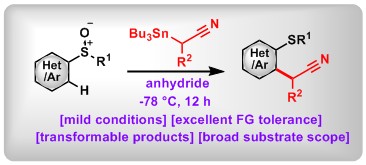

(6) Recyclable Organocatalysts for One-pot Asymmetric Synthesis of 2-Fluorocyclohexanols Bearing Six Contiguous Stereocenters. Xin Huang,* Miao Liu, Jerry P. Jasinski, Bo Peng, Wei Zhang* Adv. Synth. Catal. 2017, 359, 1919.
(5) Facile and Selective Synthesis of Imidazobenzimidazoles via a Copper-Catalysed Domino Addition/Cycloisomerisation/Coupling Process. Xu, B.;Peng, B.; Cai, B.; Wang, S.; Wang, X.; Lv, X.*Adv. Synth. Catal.2017, 358, 653.
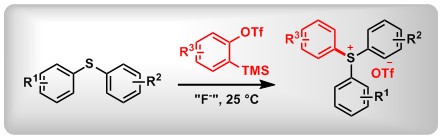
(4) Synthesis of o-Aryloxy Triarylsulfonium Salts via Aryne Insertion into Diaryl Sulfoxides. Li, X.; Sun, Y.; Huang, X.; Zhang, L.; Kong, L.; Peng, B.* Org. Lett. 2017, 19, 838.
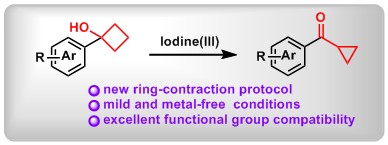
(3) Mild Ring Contractions of Cyclobutanols to Cyclopropyl Ketones via Hypervalent Iodine Oxidation. Yan Sun, Xin Huang, Xiaojin Li, Fan Luo, Lei Zhang, Mengyuan Chen, Shiya Zheng, Bo Peng* Adv. Synth. Catal.2017, 360, 1082.
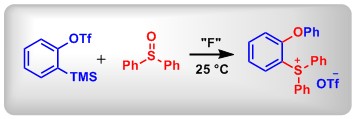
(2) Mild Synthesis of Triarylsulfonium Salts with Arynes. Zhang, L.; Li, X.; Sun, Y.; Zhao, W.; Luo, F.; Huang, X.; Lin, L.; Yang, Y. and Peng, B.* Org. Biomol. Chem., 2017, 15, 7181.
(1) Recent progress in the chemistry of keteniminium salts (review article). Li, X.; Sun, Y.; Zhang, L.; Peng, B.* Chin. J. Org. Chem. 2016, 36, 2530.
学术报告
1. 彭勃,基于烯酮亚胺盐的亲电重排反应,第十四届全国均相催化学术讨论会,大连,2015.09.22-2015.09.25
2. 彭勃,酰胺的亲电重排反应,中国化学会2015年化学青年学术论坛,福州市,2015.12.25-2015.12.28
3. 彭勃,无金属参与的有机氰化物的α芳基化,中国化学会第30届学术年会,大连,2016.07.01-2016.07.04
4. 彭勃,重排反应制备α-芳基氰化合物,中国化学会第十届全国有机化学学术会议,深圳, 2017.12.19-2017.12.21
5. 彭勃,Congestion-Accelerated [3,3]-Sigmatropic Rearrangement towards Synthesis of α-Aryl Nitriles,第十五届全国有机合成化学学术研讨会,兰州,2018.8.2-2018.8.5
6.彭勃,Aromatic Cyanoalkylation via Congestion-Accelerated [3,3]-Sigmatropic Rearrangement,第二十届全国金属有机化学学术讨论会,南京,2018.11.01-2018.11.04
7.彭勃,Fluorine Effect Enables [3,3]-Sigmatropic Rearrangement of Aryliodanes with DifluoroenolSilyl Ethers,中国高价碘化学学术研讨会,天津,2019.03.09-2019.03.10
8. 彭勃,经由σ重排的芳基亚砜邻、对位选择性C-H键烷基化,全国第十六届有机合成化学学术研讨会,开封,2019.08.08-2019.08.11
9. 彭勃,新型芳烃σ重排反应的发现,中国化学会第十一届全国有机化学学术会议,上海,2019.08.31-2019.09.03
10. 彭勃,The Rearrangement of Aryl Sulfoxides with Nitriles, 29th International Symposium on the Organic Chemistry of Sulfur, University of Guelph, Canada. 2022.07.17-2022.07.22. (online)
11. 彭勃,重访芳烃Claisen重排,中国化学会第十二届全国有机化学学术会议,合肥,2022.09.25-2022.09.29.
12. 彭勃,芳烃Claisen重排反应的新机遇,中国化学会第十七届全国有机合成化学学术会议,济南,2022.11.10-2022.11.13
博士及博士后期间代表论文及成果
1. Peng, B.; Huang, X.; Xie, L.; Maulide, N.*Angew. Chem., Int. Ed. 2014, 53, 8718. (hot paper, inside cover )
2. Peng, B.; Geerdink, D.; Farès, C.; Maulide, N.*Angew. Chem., Int. Ed. 2014, 53, 5462. (VIP paper)
3. Peng, B.; Geerdink, D.; Maulide, N.*J. Am. Chem. Soc. 2013,135, 14968.
4. Jurberg, I. D.;Peng, B.; Wöstefeld, E.; Wasserloos, M.; Maulide, N.*Angew. Chem., Int. Ed., 2012,51, 1950.
5. Huang, X.;Peng, B.; Luparia, M.; Gomes, L. F. R.; Veiros, L. F.; Maulide N.*Angew. Chem., Int. Ed., 2012,51, 8886.
6. Peng, B.; Maulide, N.*Chem. Eur. J. 2013,19, 13274.
7. Peng, B.; O’Donovan, D. H.; Jurberg, I. D.; Maulide, N.*Chem. Eur. J. 2012,18, 16292.
8. Shaaban, S.;Peng, B.; Maulide N.* Synlett.2013,24, 1722.
9. Maulide, N.;Peng, B.German patent, 2013, 13169588.4.
10. Peng, B.; Zhang, S.; Yu, X.; Feng, X.; Bao, M.*Org. Lett.2011,13, 5402.
11. Peng, B.; Feng, X.; Zhang, X.; Zhang, S.; Bao, M.*J. Org. Chem.2010,75, 2619.
12. Peng, B.; Feng, X.; Zhang, X.; Ji, L.; Bao M.*Tetrahedron2010,66, 6013.






 您的位置 :
您的位置 : 
